Epigenetic Regulation of Retinal Regeneration
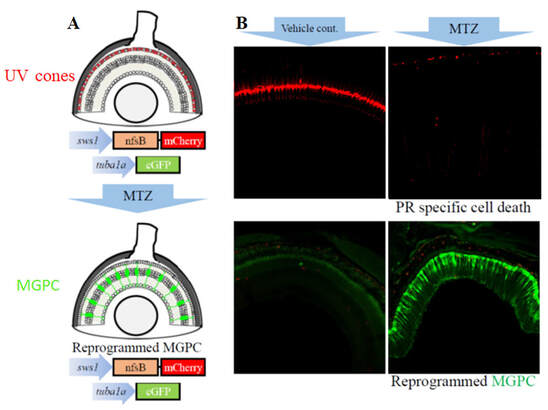
Dovetailing with our interests in epigenetic regulation of retinal development, we are also analyzing regeneration in the retina. Retinae of vertebrates possess a range of regenerative capacity; in fish and amphibians the retina is able to fully regenerate from a variety of insults but this ability is progressively lost in “higher vertebrates”, with modest regeneration in birds, and essentially no regeneration in mammals. Interestingly, the cells responsible for retinal regeneration, the Muller glia, are present in all vertebrates. In fish, retinal damage stimulates the Muller glia to reprogram into a stem cell-like state, proliferate and generate new neurons. In mammals, including humans, Muller glia similarly respond to damage but they then fail to reprogram to a stem cell-like population or generate new neurons. Instead, they proliferate and eventually form a glial scar. Why the regenerative response does not proceed in mammals as it does in fish is unknown. Due to their remarkable regenerative capacity, most studies on the molecular underpinnings of retinal regeneration have been performed in zebrafish, and a variety of factors required for Muller glia reprogramming and/or proliferation have been identified. Despite these advances, we know little about the transcriptional regulation of these genes or the epigenetic landscapes affecting their expression during regeneration. We have focused on histone modifications and have identified a role for histone acetylation and Brd4-mediated histone recognition during Muller glia reprogramming after injury (Lee and Gross, in revision).
