Morphogenesis of the eye
A major focus our research program is to elucidate the molecular and cellular control of optic cup morphogenesis, and we have focused significant effort on the choroid (optic) fissure region of the eye. During eye development, the optic primordia undergo a complex series of morphogenetic movements that ultimately result in a bilayered optic cup containing the prospective retina and retinal pigment epithelium (RPE). The neuroectodermal layers of each optic primordium must fuse along its proximo-distal axis such that the retina and RPE will be confined to the optic cup during the early phases of ocular morphogenesis. Fusion occurs at a distinct region of the optic cup called the choroid fissure (CF). Defects in CF closure result in colobomas, congenital malformations of the eye. Despite a significant amount of genetic research to identify coloboma loci, causative mutations have been identified in less than 20% of coloboma patients. Moreover, we lack a comprehensive understanding of the cell biological and morphogenetic mechanisms underlying CF closure in the human eye, in any of the animal model systems utilized for modeling human eye development and disease, or in stem cell models of optic cup formation. Previous studies in our laboratory have identified roles for the Hh pathway during optic vesicle patterning that enable CF closure (Lee et al., 2008; Lee et al., 2012), a requirement for laminin-containing extracellular matrix during CF closure (Lee et al., 2007), and we identified Bcl6a as a direct Vax2 target in the ventral optic cup, determining that it acts with several of its transcriptional co-factors (BCOR, Hdac1, Rnf2) in preventing apoptosis in the ventral optic cup during CF closure (Lee et al., 2013).
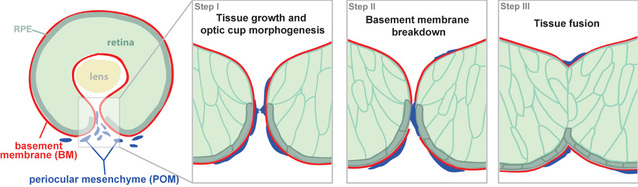
Our work has lead to a model in which choroid fissure closure occurs in three stages (Fig. 1). During Stage 1, retinoblast proliferation generates sufficient cells such that, as optic cup morphogenesis proceeds, the lateral edges of the CF are brought into close apposition. During Stage 2, the basement membrane (BM) lining the CF is degraded, and this is facilitated, in part, by the hyaloid vasculature (Hartsock et al., 2014; James et al., 2016), which then enables adhesion between cells lining the opposing sides of the fissure. During Stage 3, cells on opposing sides of the fissure form adhesions that spread and thereby close the CF. While much has been learned about Stage 1, we know virtually nothing about how BM breakdown and tissue fusion occur during CF closure, and these are the areas on which we are now focused.
Our work has lead to a model in which choroid fissure closure occurs in three stages (Fig. 1). During Stage 1, retinoblast proliferation generates sufficient cells such that, as optic cup morphogenesis proceeds, the lateral edges of the CF are brought into close apposition. During Stage 2, the basement membrane (BM) lining the CF is degraded, and this is facilitated, in part, by the hyaloid vasculature (Hartsock et al., 2014; James et al., 2016), which then enables adhesion between cells lining the opposing sides of the fissure. During Stage 3, cells on opposing sides of the fissure form adhesions that spread and thereby close the CF. While much has been learned about Stage 1, we know virtually nothing about how BM breakdown and tissue fusion occur during CF closure, and these are the areas on which we are now focused.
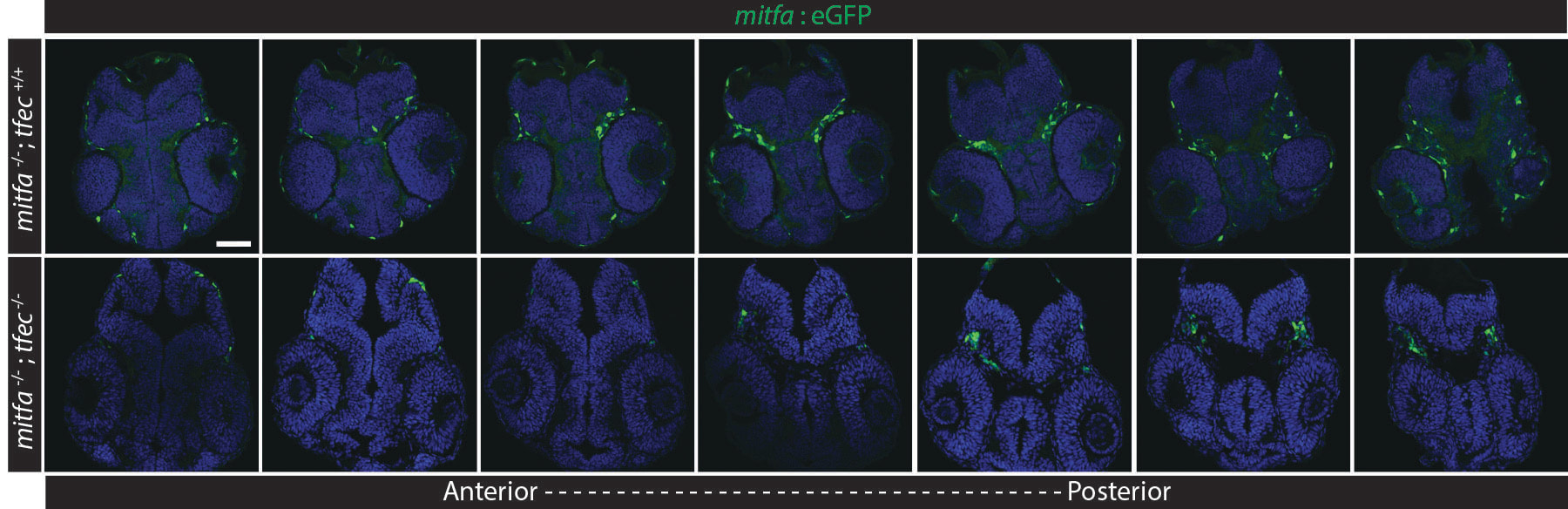
Recently, we also embarked on a study examining the function of Mitf-family transcription factors and optic cup morphogenesis, as mutations in in MITF, result in colobomas in human patients (George et al., 2016). Utilizing a novel mitfa;tfec mutant line, we first demonstrate that loss of these Mitf-family transcription factors phenocopies colobomas observed in MITF patients. Our data indicate that both RPE and cNCC development is perturbed in mitfa;tfec mutants. Through a series of embryological manipulations and rescue experiments, we determined that Mitf-family function is required specifically within the cNCCs to facilitate CF closure. Further, our data suggest that Mitf transcription factors act within cNCCs to promote localization in and around the eye and cell survival. Taken together, these data identify potential cellular underpinnings of colobomas in human COMMAD patients with mutations in MITF and provide a platform through which cNCC-specific functions during CF closure can be further elucidated (Sinagoga et al., 2020).